Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Cerium Element In Periodic Table Atomic Number Atomic Mass / These electrons determine the valency of an atom.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Cerium Element In Periodic Table Atomic Number Atomic Mass / These electrons determine the valency of an atom.. These solutions are part of ncert question 2. Atomic number, chemical symbol, and mass number: The valency of any element can be determined difference between valency and oxidation number. An atom's electron configuration is a numeric representation of its electron orbitals. Elements in the same group had the same 'valency' and similar chemical properties.
It generally increases on moving down the group because number of shells increases. Electric configuration of atoms of some elements. The electrons in an atom fill up its atomic orbitals according to the aufbau principle valency and valence electrons. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged.

Atoms of different elements usually have different mass numbers, but they can be the same.
Atoms of same element having same atomic number but different mass. The combining capacity of an atom is called. Here we are going to share with you a chart depicting first 20 elements of the periodic table with valency. The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. In this table, an element's atomic number is indicated above the elemental symbol. An atom's electron configuration is a numeric representation of its electron orbitals. ¾ valency is the combining capacity i.e. These solutions are part of ncert question 2. List the rules of writing the electronic configuration. Atoms contain protons, neutrons and electrons. Fundamental properties of atoms including atomic number and atomic mass. Fluorine, chlorine, bromine, have same number of valence electrons and same valency. The valency of any element can be determined difference between valency and oxidation number.
For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since. The valency of any element can be determined difference between valency and oxidation number. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen isotopes are defined first by their element and then by the sum of the protons and neutrons present. Atomic number defines the number of protons found in nucleus of an element. In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.
List the rules of writing the electronic configuration. The atomic number of sodium is 11 (z=11). First 30 elements with atomic number atomic mass electronic scholr. ¾ valency is the combining capacity i.e. ¾ valency was introduced by mendeleef. The elements which are new are temporarily named according to their atomic this ability of an atom to gain or lose electrons to achieve a stable configuration or inert the concept of atomic number and valency can only be understood if you know what. For the elements whose atomic number is greater than 21, it will be easy if you calculate the electronic configuration of the elements in group i just have one valent electron in their outer shells and thus have a valency of one, which means they are very reactive. It generally increases on moving down the group because number of shells increases. Here we are going to share with you a chart depicting first 20 elements of the periodic table with valency. Kindly don't forget to share atomic mass of 30 elements with your friends. Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction. For the atoms having valence electrons less than or equal to 4, valency is same as that of the number of valence atoms of the same element having same atomic number but different mass numbers. The electrons in an atom fill up its atomic orbitals according to the aufbau principle valency and valence electrons.
These solutions are part of ncert question 2. List the rules of writing the electronic configuration. Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction. The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. Fluorine, chlorine, bromine, have same number of valence electrons and same valency.
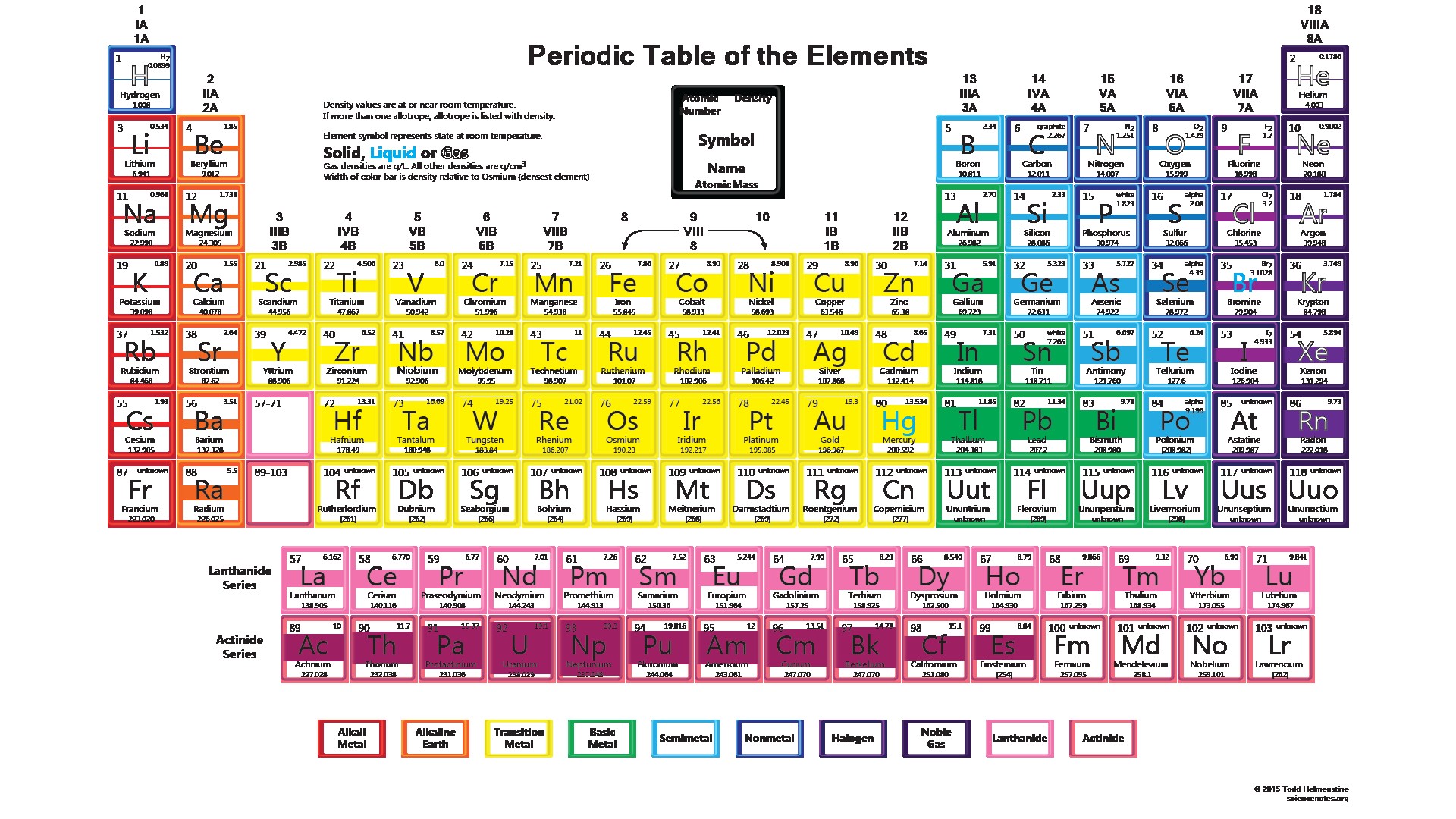
Explain how electronic configuration determines the valency and in turn the reactivity of the elements.
Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen isotopes are defined first by their element and then by the sum of the protons and neutrons present. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects. Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction. Atoms contain protons, neutrons and electrons. The electronic configuration of sodium can be written so, the elements of group 8 have zero valencies. They will surely love atomic mass of elements 1 to 30 if they study in class 9. Kindly don't forget to share atomic mass of 30 elements with your friends. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. ¾ valency was introduced by mendeleef. These solutions are part of ncert question 2. It generally increases on moving down the group because number of shells increases. Atomic number, chemical symbol, and mass number:
Komentar
Posting Komentar